AML is a highly heterogeneous disease, both genetically and phenotypically. This includes both intra- and inter-patient heterogeneity that significantly impacts prognosis and treatment responses. Mouse models are a powerful tool to further our understanding of fundamental disease biology and to advance novel therapies. While human cell line (CDX) and primary patient (PDX) xenografts are reproducible in vivo models, these models are by necessity immunodeficient. Transgenic mouse models provide an opportunity to study disease development driven by human relevant mutations within a fully immunocompetent system. However, drug development studies often require large numbers of mice and predictable time of disease development that is infeasible using primary transgenic animals. We therefore sought to develop a transgenic mouse model representative of human disease that was able to consistently be adoptively transferred into fully immunocompetent recipients.
Our laboratory has interest in multiple targeted therapeutics and chose to focus on a model that may have sensitivity to multiple translationally relevant targeted drugs. We crossed the previously established Npm1 cA (Vassiliou et al 2011), Flt3 ITD (Lee et al 2007) and Idh2 R140Q (Shih et al 2017) Cre-inducible alleles to generate a triple mutant AML model ( Npm1 cA;Idh2 R140Q;Flt3 ITD). This model develops an aggressive AML with a median survival of 44 days. Complete blood counts at endpoint demonstrate significantly elevated white cells as well as profound anemia and thrombocytopenia. Npm1 cA;Idh2 R140Q;Flt3 ITD mice also have splenomegaly and infiltration of immature myeloid cells into both lymphoid and non-lymphoid tissues. Flow cytometry analysis of the blood, spleen and bone marrow shows a significant expansion of CD11b+cKit+ cells characteristic of an AML blast phenotype.
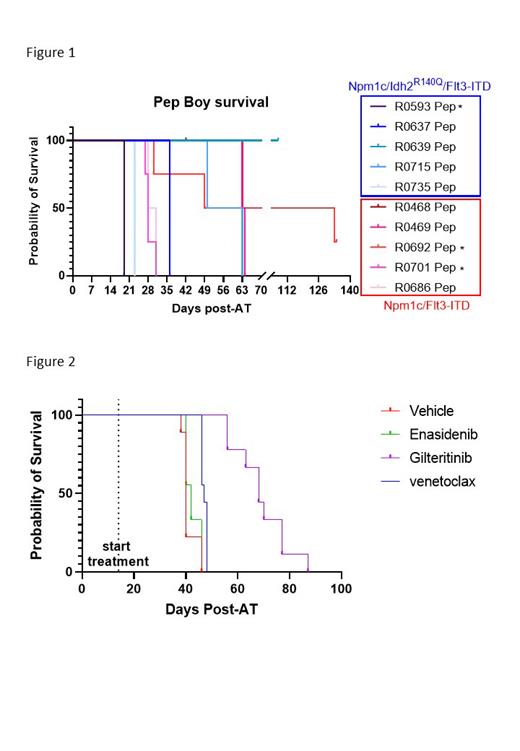
Given the aggressive nature of this model, we sought to determine whether Npm1 cA;Idh2 R140Q;Flt3 ITD AML cells could be adoptively transferred into either immunodeficient or fully immunocompetent recipients without prior preconditioning. NOD- Prkdc em26Cd52Il2rg em26Cd22/NjuCrl (NCG) and B6.SJL-Ptprca Pepcb/BoyJ (PepBoy) recipients were injected with 1x10 6 bulk splenocytes via the tail vein. Recipient mice quickly develop a lethal AML that faithfully recapitulates the disease observed in the primary transgenic donors. Circulating leukemia cells are detectable from approximately 2 weeks post adoptive transfer (post-AT) and median survival of PepBoy recipients ranged from 17 to 60 days depending on donor (Figure 1). Comprehensive immune profiling using a 36-specificity spectral flow panel showed that in PepBoy recipients, the donor derived cells accurately recapitulate the diversity of the AML immunophenotype of the primary transgenic donor. Notably, cells from male donors do not establish detectable disease in immunocompetent female recipients. This is associated with an increased number of T cells in the female recipients of male cells suggesting immune mediated control of AML development.
To assess the response to targeted therapies in a fully immune competent model, we adoptively transferred of our Npm1 cA;Idh2 R140Q;Flt3 ITD to into PepBoy recipients. Mice were randomized to treatment groups based on peripheral blood disease burden at 14 days post AT (0.2-1% of CD45+ cells). Mice received daily oral gavage of either vehicle, 100 mg/kg venetoclax (BCL2 inhibitor), 100 mg/kg enasidenib (IDH2 inhibitor) or 30 mg/kg gilteritinib (FLT3 inhibitor). All three drugs resulted in a significant reduction in circulating tumor burden after 14 days of treatment. Enasidenib did not prolong survival compared to vehicle whereas venetoclax treatment resulted in a significant survival advantage (median 47 days vs 40 in the vehicle). This model was most sensitive to gilteritinib with a median survival of 68 days. (Figure 2).
Together these findings demonstrated that the Npm1cA;Idh2 R140Q;Flt3 ITD model is an aggressive model of AML. Adoptive transfer of splenocytes into either immunodeficient NCG or fully immunocompetent PepBoy recipients faithfully recapitulates the disease of primary mice in a reproducible manner. This model therefore provides an in vivo model conducive to drug development studies and provides the opportunity to understand the impact of both AML development and therapeutics on the normal immune system.
Disclosures
Levine:Ajax: Membership on an entity's Board of Directors or advisory committees, Research Funding; Mission Bio: Membership on an entity's Board of Directors or advisory committees; Zentalis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Auron: Membership on an entity's Board of Directors or advisory committees; Prelude: Membership on an entity's Board of Directors or advisory committees; C4 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Isoplexis: Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy; Qiagen: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; AstraZeneca: Consultancy, Honoraria; Novartis: Consultancy; Roche: Honoraria; Lilly: Honoraria; Amgen: Honoraria.